A) \[{{r}_{Cs}}+{{r}_{C{{l}^{-}}}}=3a\]
B) \[{{r}_{Cs}}+{{r}_{C{{l}^{-}}}}=\frac{3a}{2}\]
C) \[{{r}_{Cs}}+{{r}_{C{{l}^{-}}}}=\frac{\sqrt{3}}{2}a\]
D) \[{{r}_{Cs}}+{{r}_{C{{l}^{-}}}}=\sqrt{3}a\]
Correct Answer: C
Solution :
[c]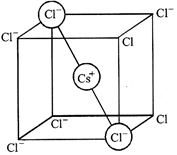 Relation between radius of cation, anion and edge length of the cube \[2{{r}_{Cs}}+2{{r}_{C{{l}^{-}}}}=\sqrt{3}a\] \[{{r}_{C{{s}^{+}}}}+{{r}_{C{{l}^{-}}}}=\frac{\sqrt{3}a}{2}\]
Relation between radius of cation, anion and edge length of the cube \[2{{r}_{Cs}}+2{{r}_{C{{l}^{-}}}}=\sqrt{3}a\] \[{{r}_{C{{s}^{+}}}}+{{r}_{C{{l}^{-}}}}=\frac{\sqrt{3}a}{2}\]
You need to login to perform this action.
You will be redirected in
3 sec
