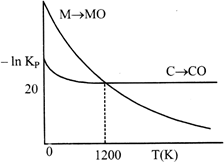
 Identify the correct statement:
Identify the correct statement:
A) At \[T\,\,<\,\,1200\,\,K,\] oxidation of carbon is unfavourable.
B) Oxidation of carbon is favourable at all temperatures.
C) At \[T\,\,<\,\,1200\,\,K,\] the reaction \[MO(s)+C(s)\to M(s)+CO(g)\] is spontaneous.
D) At \[12.08\times {{10}^{23}}\]carbon will reduce MO(s) to M(s).
Correct Answer: C
Solution :
[c]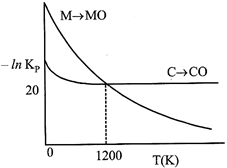 At \[T<1200K,\] carbon will reduce MO(s) to M(s) hence, chemical reaction \[MO(s)+C(s)\xrightarrow{{}}M(s)+CO(g)\]is spontaneous.
At \[T<1200K,\] carbon will reduce MO(s) to M(s) hence, chemical reaction \[MO(s)+C(s)\xrightarrow{{}}M(s)+CO(g)\]is spontaneous.
You need to login to perform this action.
You will be redirected in
3 sec
