| Column-I | Column-II | ||
| [A] | Aniline | (i) | Red colour with \[FeC{{l}_{3}}\] |
| [B] | Benzene sulfonic acid | (ii) | Violet colour with sodium |
| [C] | Thiourea | (iii) | Blue colour with hot and acidic solution of \[FeS{{O}_{4}}\] |
A) A\[\to \](ii); B\[\to \](iii); C\[\to \](i)
B) A\[\to \](iii); B\[\to \](i); C\[\to \](ii)
C) A\[\to \](iii); B\[\to \](ii); C\[\to \](i)
D) A\[\to \](ii); B\[\to \](i); C\[\to \](iii)
Correct Answer: B
Solution :
[b] In Lassaigne's test, fusion with sodium take place and following species formed respectively. [a] Aniline \[\xrightarrow{{}}C{{N}^{-}}\] [b] Benzene sulfonic acid \[\xrightarrow{{}}\]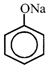 [c] Thiourea \[\xrightarrow{{}}{{S}^{2-}}\] Reaction of \[C{{N}^{-}}\] with hot and acidic solution of \[FeS{{O}_{4}}\] lead to formation of \[F{{e}_{4}}{{[FeCN)}_{6}}{{]}_{3}}\] which is blue in colour. It contains iron in both II and III oxidation state. Reaction of \[{{S}^{2-}}\] with sodium nitroprusside \[N{{a}_{2}}S+N{{a}_{2}}[Fe{{(CN)}_{5}}NO]\xrightarrow{{}}\underset{(violet\,\,in\,\,colour)}{\mathop{N{{a}_{4}}[Fe{{(CN)}_{5}}NOS]}}\,\] Phenoxide ion on reacting with \[FeC{{l}_{3}}\] give red colour with \[FeC{{l}_{3}}.\]
[c] Thiourea \[\xrightarrow{{}}{{S}^{2-}}\] Reaction of \[C{{N}^{-}}\] with hot and acidic solution of \[FeS{{O}_{4}}\] lead to formation of \[F{{e}_{4}}{{[FeCN)}_{6}}{{]}_{3}}\] which is blue in colour. It contains iron in both II and III oxidation state. Reaction of \[{{S}^{2-}}\] with sodium nitroprusside \[N{{a}_{2}}S+N{{a}_{2}}[Fe{{(CN)}_{5}}NO]\xrightarrow{{}}\underset{(violet\,\,in\,\,colour)}{\mathop{N{{a}_{4}}[Fe{{(CN)}_{5}}NOS]}}\,\] Phenoxide ion on reacting with \[FeC{{l}_{3}}\] give red colour with \[FeC{{l}_{3}}.\]
You need to login to perform this action.
You will be redirected in
3 sec
