A) \[0.8\times {{10}^{-5}}\]
B) \[1.79\times {{10}^{-5}}\]
C) \[0.182\times {{10}^{-5}}\]
D) none of the above
Correct Answer: C
Solution :
[c] Percentage of degree of ionization = 1.34% \[\therefore \] Degree of ionization \[(\alpha )=0.0134\]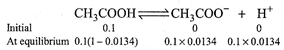 \[K=\frac{[C{{H}_{3}}CO{{O}^{-}}][{{H}^{+}}]}{[C{{H}_{3}}COOH]}\] \[=\frac{0.1\times 0.0134\times 0.0134\times 0.1}{0.1\times (1-0.0134)}\] \[=0.182\times {{10}^{-5}}\]
\[K=\frac{[C{{H}_{3}}CO{{O}^{-}}][{{H}^{+}}]}{[C{{H}_{3}}COOH]}\] \[=\frac{0.1\times 0.0134\times 0.0134\times 0.1}{0.1\times (1-0.0134)}\] \[=0.182\times {{10}^{-5}}\]
You need to login to perform this action.
You will be redirected in
3 sec
