A) \[109{}^\circ 28'\]
B) \[100{}^\circ \]
C) \[180{}^\circ \]
D) \[120{}^\circ \]
Correct Answer: D
Solution :
[d] All the properties mentioned in the question suggest that it is a benzene molecule. Since in benzene all carbons are \[s{{p}^{2}}-\]hybridized, therefore, C - C - C angle is \[120{}^\circ \]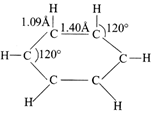
You need to login to perform this action.
You will be redirected in
3 sec
