A) equal to that of forward reaction.
B) less than that of forward reaction.
C) greater than that of forward reaction.
D) given values are not sufficient to explain given statement.
Correct Answer: C
Solution :
[c] K decreases with increase in temperature. Thus, forward reaction is exothermic.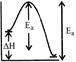 \[{{E}_{a}}=\] activation energy of forward reaction \[{{E}_{a}}'=\]... backward... \[=({{E}_{a}}+\Delta H)\] Thus, \[E_{a}^{'}>{{E}_{a}}\]
\[{{E}_{a}}=\] activation energy of forward reaction \[{{E}_{a}}'=\]... backward... \[=({{E}_{a}}+\Delta H)\] Thus, \[E_{a}^{'}>{{E}_{a}}\]
You need to login to perform this action.
You will be redirected in
3 sec
