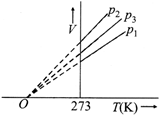
 What is the correct order of pressures?
What is the correct order of pressures?
A) \[{{p}_{1}}>{{p}_{3}}>{{p}_{2}}\]
B) \[{{p}_{1}}>{{p}_{2}}>{{p}_{3}}\]
C) \[{{p}_{2}}>{{p}_{3}}>{{p}_{1}}\]
D) \[{{p}_{2}}>{{p}_{1}}>{{p}_{3}}\]
Correct Answer: A
Solution :
[a] From the graph we can see the correct order of pressures \[{{p}_{1}}>{{p}_{3}}>{{p}_{2}}\]You need to login to perform this action.
You will be redirected in
3 sec
