A) \[HCOOH>C{{H}_{3}}COOH>ClC{{H}_{2}}COOH\]\[>{{C}_{2}}{{H}_{5}}COOH\]
B) \[ClC{{H}_{2}}COOH>HCOOH>C{{H}_{3}}COOH\]\[>{{C}_{2}}{{H}_{5}}COOH\]
C) \[C{{H}_{3}}COOH>HCOOH>ClC{{H}_{2}}COOH\]\[>{{C}_{2}}{{H}_{5}}COOH\]
D) \[{{C}_{2}}{{H}_{5}}COOH>C{{H}_{3}}COOH>HCOOH\]\[>ClC{{H}_{2}}COOH\]
Correct Answer: B
Solution :
[b] Recall that presence of electron- withdrawing group increases, while presence of electron-releasing group decreases the acidity of carboxylic acids.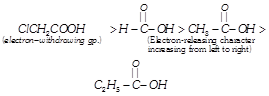
You need to login to perform this action.
You will be redirected in
3 sec
