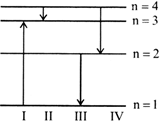
A) IV
B) III
C) II
D) I
Correct Answer: B
Solution :
[b] \[E=Rhc\left[ \frac{1}{{{n}_{1}}^{2}}-\frac{1}{{{n}_{2}}^{2}} \right]\] E will be maximum for the transition for which \[\left[ \frac{1}{{{n}_{1}}^{2}}-\frac{1}{{{n}_{2}}^{2}} \right]\] is maximum. Here \[{{n}_{2}}\] is the higher energy level. Clearly, \[\left[ \frac{1}{{{n}_{1}}^{2}}-\frac{1}{{{n}_{2}}^{2}} \right]\] is maximum for the third transaction, i.e. \[2\to 1.\]I transition represents the absorption of energy.You need to login to perform this action.
You will be redirected in
3 sec
