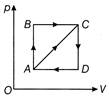
 \[{{p}_{A}}=3\times {{10}^{4}}\,Pa;\,\,{{V}_{A}}=2\times {{10}^{-\,3}}\,{{m}^{3}}\] \[{{p}_{B}}=8\times {{10}^{4}}\,Pa;\,\,{{V}_{D}}=5\times {{10}^{-\,3}}\,{{m}^{3}}\] In the process AB, 600 J of heat is added to the system and in process BC, 200 J of heat is added to the system. The change in internal energy of the system in process AC would be
\[{{p}_{A}}=3\times {{10}^{4}}\,Pa;\,\,{{V}_{A}}=2\times {{10}^{-\,3}}\,{{m}^{3}}\] \[{{p}_{B}}=8\times {{10}^{4}}\,Pa;\,\,{{V}_{D}}=5\times {{10}^{-\,3}}\,{{m}^{3}}\] In the process AB, 600 J of heat is added to the system and in process BC, 200 J of heat is added to the system. The change in internal energy of the system in process AC would be
A) 560 J
B) 800 J
C) 600 J
D) 640 J
Correct Answer: A
Solution :
Since AB is isochoric process, so no work is done, i.e., \[{{W}_{AB}}=0\] BC is isobaric process \[{{W}_{BC}}={{P}_{B}}({{V}_{D}}-{{V}_{A}})=240J\] Therefore, \[\Delta Q = 600 + 200 = 800 J\] Using \[\Delta Q=\,\,\Delta U+\Delta W\] \[\Rightarrow \,\,\,\,\Delta U=\,\,\Delta Q-\Delta W\,\,=\,\,800-\,\,240\,\,=\,\,560\,J\]You need to login to perform this action.
You will be redirected in
3 sec
