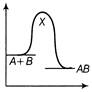
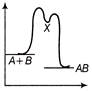
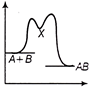
| An exothermic chemical reaction occurs in two steps as follows |
| I. \[A+B\,\,\to \,\,X\,(fast)\] |
| II. \[X\,\to \,\,AB\,(slow)\] |
| The progress of the reaction can be best represented by |
A)
B)
C)
D) All of these
Correct Answer: C
Solution :
The reaction occuring in two steps has two activation energy peaks. The first step being fast needs less activation energy. The second step being slow, needs more activation energy. Therefore, second peak will be higher than the first.You need to login to perform this action.
You will be redirected in
3 sec
