A) pentagonal bipyramidal
B) octahedral
C) square pyramidal
D) octahedral monopyramidal
Correct Answer: A
Solution :
Structure of \[{{\operatorname{IF}}_{7}}\] is determined by using hybridisation as follows \[H=\frac{V+M-C+A}{2}\,\,=\,\,\frac{7+7}{2}\,\,=\,\,7\] \[\operatorname{Hybridisation} =s{{p}^{3}}{{d}^{3}}\] \[\operatorname{Structure} = Pentagonal bipyramidal\]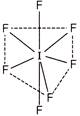
You need to login to perform this action.
You will be redirected in
3 sec
