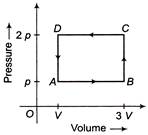
A) 4pV
B) 2pV
C) \[\frac{pV}{2}\]
D) pV
Correct Answer: B
Solution :
Work done in cyclic process is equal to the area under the cycle, also work done is positive, if cycle is clockwise and negative if cycle is anticlockwise. In cyclic process \[\Delta U = 0\] By 1st law of thermodynamics \[\Delta O=\Delta U+\Delta W\] ... (i) \[\Delta W=-Area of rectangle\,\,ABCD\] \[=-p(2V)=-\,2pV\] Putting \[\Delta U= 0 \,and\,\,\Delta W = -2 pV\] in Eq. (i) We have \[\Delta Q = \Delta W = -2pV\] (AO heat absorbed) \[\therefore \,\,\,\,Heat rejected by gas =2p\,V\]You need to login to perform this action.
You will be redirected in
3 sec
