A) 4
B) 3
C) 2
D) 1
Correct Answer: A
Solution :
In ice each water molecule forms four hydrogen bond through which each water molecule is tetrahedrally attached with other water molecule.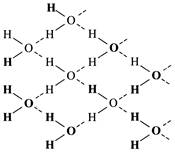
You need to login to perform this action.
You will be redirected in
3 sec
