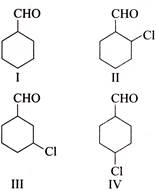
A) \[\left( I \right)>\left( II \right)>\left( in \right)>\left( IV \right)\]
B) \[\left( IV \right) > \left( III \right) >\left( II \right)> \left( I \right)\]
C) \[\left( II \right) > \left( III \right) > \left( IV \right) > \left( I \right)\]
D) \[\left( I \right) > \left( IV \right) > \left( III \right) > \left( II \right)\]
Correct Answer: C
Solution :
All are aldehydes, -I effect of Cl at \[C-2>\] at\[C-3\] at \[\operatorname{C} - 4\]. The more the -I effect, the more reactive is the aldehyde towards NA reaction\[\left[ \left( II \right) > \left( III \right) > \left( IV \right) > \left( I \right) \right]\].You need to login to perform this action.
You will be redirected in
3 sec
