A) \[{{\lambda }_{3}}=\,\,{{\lambda }_{1}}\,+\,{{\lambda }_{2}}\]
B) \[{{\lambda }_{3}}=\,\,\frac{{{\lambda }_{1}}\,{{\lambda }_{2}}}{{{\lambda }_{1}}+{{\lambda }_{2}}}\]
C) \[{{\lambda }_{1}}+{{\lambda }_{2}}+{{\lambda }_{3}}=0\]
D) \[{{\lambda }_{3}}^{2}+{{\lambda }^{2}}_{1}\,+\lambda _{2}^{2}\]
Correct Answer: B
Solution :
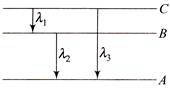
Let the energy in A, B and C state be\[{{E}_{A}},\,\,{{E}_{B}}\,and\,\,{{E}_{C}}\], then from the figure \[\left( {{E}_{C}}-{{E}_{B}} \right)+\left( {{E}_{B}}-{{E}_{A}} \right)=\left( {{E}_{C}}-{{E}_{A}} \right)\] \[\frac{hc}{{{\lambda }_{1}}}+\frac{hc}{{{\lambda }_{2}}}=\frac{hc}{{{\lambda }_{3}}}\] \[\Rightarrow \,\,\,\,\,\,\,{{\lambda }_{3}}=\frac{{{\lambda }_{1}}{{\lambda }_{2}}}{{{\lambda }_{1}}+{{\lambda }_{2}}}\]
You need to login to perform this action.
You will be redirected in
3 sec
