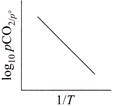
A)
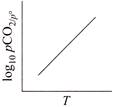
B)
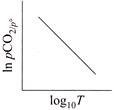
C)
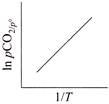
D)
Correct Answer: A
Solution :
For the reaction, \[CaC{{O}_{3\,(g)}}\,\,\rightleftharpoons \,\,Ca{{O}_{(s)}}+C{{O}_{2}}_{\,(g)}\] \[{{K}_{p}}={{P}_{C{{O}_{2}}}},and\,\,{{K}_{C}}\,\,=\,\,[C{{O}_{2}}]\] \[(\because \,\, \left[ CaC{{O}_{3\,(g)}} \right] =1 and \left[ CaO \right]=1 for solids]\] According to Arrhenius equation, we have \[K=A{{e}^{-\Delta H_{r}^{{}^\circ }/RT}}\] Taking logarithm, we have \[\log \,{{K}_{p}}=\log \,A-\frac{\Delta H_{^{r}}^{o}}{RT\,\,(2.303)}\] This is an equation of straight line. When log \[{{K}_{p}}\] is plotted against 1/T, we get a straight line. \[\operatorname{The} intercept of this line = log\,A\] \[\operatorname{slope} =-\Delta H_{r}^{{}^\circ }/\,\,2.303\,\,R\]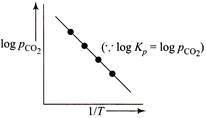 Knowing the value of slope from the plot and universal gas constant R, \[\Delta H_{r}^{{}^\circ }\] can be calculated. Equation of straight line: \[\operatorname{Y}= mx\,+ C\]. Here, \[\log \,\,{{K}_{p}}=-\frac{\Delta H_{r}^{o}}{2.303\,R}\,\left( \frac{1}{T} \right)+\log \,A\]
Knowing the value of slope from the plot and universal gas constant R, \[\Delta H_{r}^{{}^\circ }\] can be calculated. Equation of straight line: \[\operatorname{Y}= mx\,+ C\]. Here, \[\log \,\,{{K}_{p}}=-\frac{\Delta H_{r}^{o}}{2.303\,R}\,\left( \frac{1}{T} \right)+\log \,A\]
You need to login to perform this action.
You will be redirected in
3 sec
