A) \[{{\lambda }_{3}}=\,\,{{\lambda }_{1}}+\,\,{{\lambda }_{2}}\]
B) \[{{\lambda }_{3}}=\,\,\frac{{{\lambda }_{1}}{{\lambda }_{2}}}{{{\lambda }_{1}}+{{\lambda }_{2}}}\]
C) \[{{\lambda }_{1}}+{{\lambda }_{2}}+{{\lambda }_{3}}=0\]
D) \[\lambda _{3}^{2}\,=\,\lambda _{1}^{2}\,\,+\,\lambda _{2}^{2}\]
Correct Answer: B
Solution :
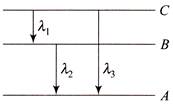
Let the energy in A, B and C state be\[{{E}_{A}},\,\,{{E}_{B}}\,and\,\,{{E}_{C}}\] then from the figure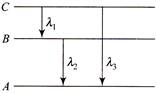 \[{{v}_{Balmer}}\,\,=\,\,\frac{c}{{{\lambda }_{\max }}}\,\,=\,\,Rc\,\left[ \frac{1}{{{(2)}^{2}}}-\frac{1}{{{(3)}^{2}}} \right]\,\,=\,\,\frac{5\,RC}{36}\] \[or\,\,\,\,\,\frac{hc}{{{\lambda }_{1}}}+\frac{hc}{{{\lambda }_{2}}}=\frac{hc}{{{\lambda }_{3}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,{{\lambda }_{3}}\,=\,\frac{{{\lambda }_{1}}{{\lambda }_{2}}}{{{\lambda }_{1}}+{{\lambda }_{2}}}\]
\[{{v}_{Balmer}}\,\,=\,\,\frac{c}{{{\lambda }_{\max }}}\,\,=\,\,Rc\,\left[ \frac{1}{{{(2)}^{2}}}-\frac{1}{{{(3)}^{2}}} \right]\,\,=\,\,\frac{5\,RC}{36}\] \[or\,\,\,\,\,\frac{hc}{{{\lambda }_{1}}}+\frac{hc}{{{\lambda }_{2}}}=\frac{hc}{{{\lambda }_{3}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,{{\lambda }_{3}}\,=\,\frac{{{\lambda }_{1}}{{\lambda }_{2}}}{{{\lambda }_{1}}+{{\lambda }_{2}}}\]
You need to login to perform this action.
You will be redirected in
3 sec
