A) \[4\,\,{{P}_{O}}{{V}_{O}}\]
B) \[6\,\,{{P}_{O}}{{V}_{O}}\]
C) \[4.5\,\,{{P}_{O}}{{V}_{O}}\]
D) \[2\,\,{{P}_{O}}{{V}_{O}}\]
Correct Answer: B
Solution :
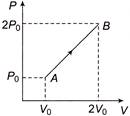
Change in internal energy from \[A\,\,\to \,\,B\] is \[\Delta U\,\,=\,\,\frac{f}{2}\,\mu R\Delta T\,\,=\,\,\frac{f}{2}\,({{P}_{f}}{{V}_{f}}-{{P}_{i}}{{V}_{i}})\] \[=\,\,\frac{3}{2}\,(2{{P}_{0}}\times 2{{V}_{0}}-{{P}_{0}}\times {{V}_{0}})\,\,=\,\,\frac{9}{2}\,{{P}_{0}}{{V}_{0}}\,\,\] Work done in process \[A\,\,\to \,\,B\] is equal to the area covered by the graph with volume axis i.e., \[{{W}_{A\to B}}\,\,=\,\,\frac{1}{2}\,({{P}_{0}}+2{{P}_{0}})\,\times \,(2{{V}_{0}}-{{V}_{0}})\,\,=\,\,\,\frac{3}{2}\,{{P}_{0}}{{V}_{0}}\] Hence, \[\Delta Q\,\,=\,\,\Delta U+\Delta W\,\,=\,\,\frac{9}{2}\,{{P}_{0}}{{V}_{0}}\,+\frac{3}{2}{{P}_{0}}{{V}_{0}}=\,\,6{{P}_{0}}{{V}_{0}}\,\,\]You need to login to perform this action.
You will be redirected in
3 sec
