A) Lone pair - bond pair repulsion only
B) Bond pair - bond pair repulsion only
C) Lone pair - lone pair repulsion and lone pair bond pair repulsion
D) Lone pair - lone pair repulsion only
Correct Answer: D
Solution :
[d] Lone pair-lone pair repulsion only \[Br{{F}_{3}}\]molecule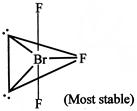 In this molecule lone pair decide the shape. It has two lone pairs and three bond pairs based on Ip-lp, Ip-bp and bp-bp repulsion it is found that it has least repulsion.
In this molecule lone pair decide the shape. It has two lone pairs and three bond pairs based on Ip-lp, Ip-bp and bp-bp repulsion it is found that it has least repulsion.
You need to login to perform this action.
You will be redirected in
3 sec
