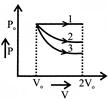
A) \[\Delta {{U}_{1}}>\Delta {{U}_{2}}>\Delta {{U}_{3}}\]
B) \[\Delta {{U}_{2}}<\Delta {{U}_{1}}<\Delta {{U}_{3}}\]
C) \[\Delta {{U}_{1}}<\Delta {{U}_{2}}<\Delta {{U}_{3}}\]
D) \[\Delta {{U}_{2}}<\Delta {{U}_{3}}<\Delta {{U}_{1}}\]
Correct Answer: C
Solution :
[c] \[\Delta {{U}_{2}},\Delta {{U}_{1}}<\Delta {{U}_{3}}\]You need to login to perform this action.
You will be redirected in
3 sec
