A) \[-\frac{{{E}_{a}}}{2.303}\]
B) \[-\frac{{{E}_{a}}}{2.303\,R}\]
C) \[-\frac{2.303}{{{E}_{a}}\,R}\]
D) \[-\frac{{{E}_{a}}\,}{R}\]
Correct Answer: B
Solution :
\[\because \] \[\log \,K=\log A-\frac{{{E}_{n}}}{2.303\,RT}\] When a graph is plotted between log K and %, for first order reaction a straight line is obtained and the slope of the line is equal to \[-\frac{{{E}_{n}}}{2.303\,R}\]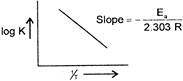
You need to login to perform this action.
You will be redirected in
3 sec
