A) \[{{\operatorname{NO}}^{+}}_{2}> N{{O}_{2}}> N{{O}^{-}}_{2}\]
B) \[{{\operatorname{NO}}^{-}}_{2}> N{{O}_{2}}> N{{O}^{+}}_{2}\]
C) \[{{\operatorname{NO}}_{2}}> N{{O}_{2}}^{-}>N{{O}^{+}}_{2}\]
D) \[{{\operatorname{NO}}_{2}}> N{{O}_{2}}^{-}>N{{O}^{+}}_{2}\]
Correct Answer: A
Solution :
\[{{\operatorname{NO}}^{+}}_{2} > N{{O}_{2}} > N{{O}^{-}}_{2}\] Because has no unshared electron and hence it is linear \[N{{O}_{2}}\]has one unshared electron while has one unshared electron pair.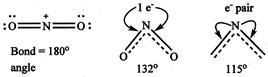
You need to login to perform this action.
You will be redirected in
3 sec
