A) -5 J
B) -10 J
C) -15 J
D) -20 J
Correct Answer: A
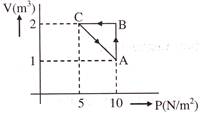
Solution :
\[\Delta {{W}_{AB}}\,\,=\,\,P\Delta V\text{ }=\text{ }\left( 10 \right)\text{ }\left( 2-1 \right)=10\text{ }J\] \[\Delta {{W}_{BC}}\,\,=\,\,0\] From first law of thermodynamics \[\Delta Q=\Delta W+\Delta U\] \[AU\text{ }=\text{ }0\] (process ABCA is cyclic) \[\therefore \,\,\,\,\Delta Q\,\,=\,\,\,\Delta {{W}_{AB}}+\Delta {{W}_{BC}}+\Delta {{W}_{CA}}\] \[\therefore \,\,\,\text{ }\Delta {{W}_{CA}}=\,\,\Delta Q\text{ }-\Delta {{W}_{AB}}-\text{ }\Delta {{W}_{BC}}=5\,\,-\,\,10\,\,-\,\,0\]You need to login to perform this action.
You will be redirected in
3 sec
