A) Both complexes have square planar geometries
B) Both complexes have tetrahedral geometries
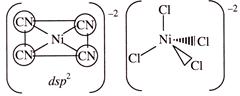
C) \[{{\left[ NiC{{l}_{4}} \right]}^{2-}}\] has a square planar geometry while \[{{\left[ Ni{{(CN)}_{4}} \right]}^{2-}}\] has a tetrahedral geometry
D) \[{{\left[ NiC{{l}_{4}} \right]}^{2-}}\] has a tetrahedral geometry while \[{{\left[ Ni{{(CN)}_{4}} \right]}^{2-}}\] has a square planar geometry
Correct Answer: D
Solution :
You need to login to perform this action.
You will be redirected in
3 sec
