A) \[1.815\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
B) \[2.8\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
C) \[3.8\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
D) \[4.815\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
Correct Answer: A
Solution :
\[2{{r}_{Cl-}}\,=\,\,\frac{\sqrt{2}}{2}\,\,\times \,\,a\,\,=\,\frac{\sqrt{2}}{4}\,\,\times \,\,5.14\,\,=\,\,1.815\]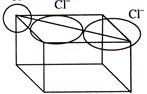
You need to login to perform this action.
You will be redirected in
3 sec
