A) \[{{\operatorname{NO}}_{2}} and C{{O}_{2}}\]
B) \[{{\operatorname{NO}}_{2}} and {{O}_{3}}\]
C) \[{{\operatorname{SiF}}_{2}} and C{{O}_{2}}\]
D) \[{{\operatorname{SiF}}_{4}} and N{{O}_{2}}\]
Correct Answer: B
Solution :
Dipole moment = Product of magnitude of - ve or +ve charge q and distance d between the centres of +ve and -ve charges. \[\mu =q\times d\] Dipole moment of: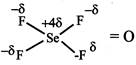 While \[{{\operatorname{NO}}_{2}} and {{O}_{3}}\]are bent molecules with permanent dipole moment.
While \[{{\operatorname{NO}}_{2}} and {{O}_{3}}\]are bent molecules with permanent dipole moment.
You need to login to perform this action.
You will be redirected in
3 sec
