A) 380 J
B) 500 J
C) 460 J
D) 300 J
Correct Answer: C
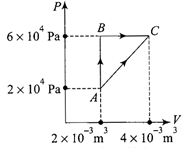
Solution :
In cyclic process \[{{Q}_{cyclic}}={{W}_{cyclic}}\] \[{{Q}_{AB}}+{{A}_{BC}}+{{Q}_{CA}}=\text{area}\,\text{enclosed}\] \[400+100+{{Q}_{CA}}=\frac{1}{2}\times \left( 4\times {{10}^{4}}\times 2\times {{10}^{-3}} \right)\] \[{{Q}_{AC}}=500-40\,J\,=460\,J\]You need to login to perform this action.
You will be redirected in
3 sec
