A) Pentagonal bipyramidal
B) Tetrahedral
C) Trigonal bipyramidal
D) Octahedral
Correct Answer: A
Solution :
Number of electron pairs around iodine in \[\text{I}{{\text{F}}_{\text{7}}}\]molecule is seven so the hybridization is \[s{{p}^{3}}{{d}^{3}}\]and the shape (geometry) is pentagonal bipyramidal.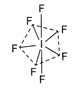 Hence, the correct option is (a).
Hence, the correct option is (a).
You need to login to perform this action.
You will be redirected in
3 sec
