| \[Z{{n}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Zn;E=-7.62\,V,\] |
| \[F{{e}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Fe;E=-7.81\,V,\] |
| The emf of the cell \[F{{e}^{2+}}+Zn\xrightarrow{{}}Z{{n}^{2+}}+Fe\]is |
A) 1.54 V
B) -1.54 V
C) -0.19 V
D) +0.19 V
Correct Answer: C
Solution :
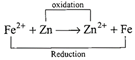 \[EMF={{E}_{cathode}}-Eanode=-7.81-(-7.62)\] \[=EMF=-0.19\,V.\]
\[EMF={{E}_{cathode}}-Eanode=-7.81-(-7.62)\] \[=EMF=-0.19\,V.\]
You need to login to perform this action.
You will be redirected in
3 sec
