| For the following parallel chain reaction |
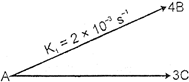 |
| what will be that value of overall half-life of A in minutes?\[\left[ \text{Given}\,\,\text{thet}\,\,\frac{{{[B]}_{t}}}{{{[C]}_{t}}}=\frac{16}{9} \right]\] |
A) 3.3
B) 6.3
C) 3.6
D) None
Correct Answer: A
Solution :
| We have \[\frac{{{[B]}_{t}}}{[C]}=\frac{4{{k}_{1}}}{3{{k}_{2}}}=\frac{16}{9}\] so \[\frac{{{k}_{1}}}{{{k}_{2}}}=\frac{4}{3}\] now \[k={{k}_{1}}+{{k}_{2}}=[2\times {{10}^{-\,3}}+\frac{3}{4}\times 2\times {{10}^{-\,3}}]{{\sec }^{-1}}\] \[=\frac{7}{2}\times {{10}^{-\,3}}{{\sec }^{-1}}=\frac{7\times {{10}^{-\,3}}\times 60}{2}{{\min }^{-1}}\] so \[{{T}_{1/2}}=\frac{\ell n\,2}{7\times 30\times {{10}^{-\,3}}}\min =\frac{693}{7\times 30}=3.3\min \] |
You need to login to perform this action.
You will be redirected in
3 sec
