A) nickel is in the same oxidation state in both
B) both are paramagnetic in nature
C) have square planar and tetrahedral geometry respectively
D) have tetrahedral and square planar geometry Respectively
Correct Answer: D
Solution :
| In\[\left[ Ni{{(CO)}_{4}} \right]\], \[Ni\]is in zero oxidation state. Its electronic configuration is\[\left[ Ar \right]3{{d}^{4}}4{{s}^{2}}\]. Pairing of electrons occurs due to strong field ligand |
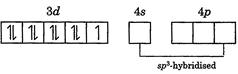 |
| It has tetrahedral geometry and is diamagnetic (as all the electrons are paired). |
| The oxidation state of \[Ni\]in \[{{\left[ Ni{{\left( CN \right)}_{4}} \right]}^{2-}}\] is \[+2\]. It's electronic configuration is \[3{{d}^{8}}4{{s}^{0}}\]. Pairing of electrons will also occur in this case as \[C{{N}^{-}}\]is a strong field ligand. |
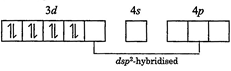 |
| It has square planar geometry and is diamagnetic in nature. |
You need to login to perform this action.
You will be redirected in
3 sec
