A) 2
B) 1
C) 0
D) 3
Correct Answer: A
Solution :
Idea This problem includes conceptual mixing of degree of unsaturation of organic compound, Wurtz reaction. Determine the structural formula of compound using degree of un saturation. Complete the chemical reaction again calculate degree of un saturation of product. Determination of molecular structure of \[{{C}_{4}}{{H}_{6}}ClBr\]Degree of un saturation (u) \[u=(C+1)-\frac{H}{2}+\frac{T}{2}=(4+1)-\frac{8}{2}=1\] According to question the compound is cyclic means the compound contain 4 carbon is cyclobutane derivative having Cl and Br are at diagonal position. Hence, the structure of compound is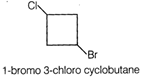 This compound on reaction with metallic sodium produces bicyclic product as follows
This compound on reaction with metallic sodium produces bicyclic product as follows 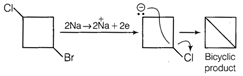 Saturated monocyclic compound has u =1 Saturated bicyclic compound has u = 2 TEST Edge Problem including Corey House reaction Wurtz reaction, Kolbey reaction and degree of unsaturation are also asked frequently. So, students should go through it.
Saturated monocyclic compound has u =1 Saturated bicyclic compound has u = 2 TEST Edge Problem including Corey House reaction Wurtz reaction, Kolbey reaction and degree of unsaturation are also asked frequently. So, students should go through it.
You need to login to perform this action.
You will be redirected in
3 sec
