| Direction: Assertion- Reaction type. Each of these contains tow statements: Statement I (Assertion), Statement II (Reason). Each of these questions also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below: |
| Statement I: N-atom in \[N{{H}_{3}}\] is \[s{{p}^{3}}\]hybridized and bond angle is \[107{}^\circ \]. |
| Statement II: \[lp-bp\] repulsion (VSEPR) decreases bond angle to \[107{}^\circ \]. |
A) Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I,
B) Statement I is true; Statement II is false.
C) Statement I is false; Statement II is true.
D) Statement I is true; Statement II is true; Statements is the correct explanation for Statement I.
Correct Answer: D
Solution :
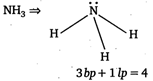 ie,\[3bp+1lp=4\]thus, hybridisation is \[s{{p}^{3}}\] Ammonia contains both bond pair of electrons and lone pair of electrons, thus there are Ip - bp and bp-bp repulsions. The presence of lone-pair cause slight distortion in bond angle from \[109{}^\circ 28\text{ }to\text{ }107{}^\circ 48\] and structure becomes pyramidal.
ie,\[3bp+1lp=4\]thus, hybridisation is \[s{{p}^{3}}\] Ammonia contains both bond pair of electrons and lone pair of electrons, thus there are Ip - bp and bp-bp repulsions. The presence of lone-pair cause slight distortion in bond angle from \[109{}^\circ 28\text{ }to\text{ }107{}^\circ 48\] and structure becomes pyramidal.
You need to login to perform this action.
You will be redirected in
3 sec
