A) \[He\] and \[Ar\]
B) \[He\] and \[{{O}_{2}}\]
C) \[{{O}_{2}}\] and \[{{N}_{2}}\]
D) \[{{O}_{2}}\] and \[He\]
Correct Answer: D
Solution :
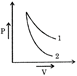
In the graph given, slope of curve 2 is greater than the slope of curve 1. \[{{\left( \frac{\gamma P}{V} \right)}_{2}}>{{\left( \frac{\gamma P}{V} \right)}_{1}}\Rightarrow {{\gamma }_{2}}>{{\gamma }_{1}}\]\[{{\gamma }_{He}}>{{\gamma }_{{{O}_{2}}}}\] Since, \[{{\gamma }_{monoatomic}}>{{\gamma }_{diatomic}}\] Hence, curve 2 corresponds to helium and curve 1 corresponds to oxygen.You need to login to perform this action.
You will be redirected in
3 sec
