A) \[4\,{{N}_{A}}\]
B) \[0.4\,{{N}_{A}}\]
C) \[0.1\,{{N}_{A}}\]
D) \[0.2\,{{N}_{A}}\]
Correct Answer: B
Solution :
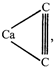 \[6.4g\] of \[Ca{{C}_{2}}\] contain \[\pi \]-electron \[=4{{N}_{2}}\]
\[6.4g\] of \[Ca{{C}_{2}}\] contain \[\pi \]-electron \[=4{{N}_{2}}\]
You need to login to perform this action.
You will be redirected in
3 sec
