A) \[5\,kJ\]
B) \[25\,kJ\]
C) \[15\,kJ\]
D) \[20\,kJ\]
Correct Answer: C
Solution :
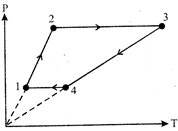
\[\text{Proces}{{\text{s}}_{1\to 2}}\] and \[\text{Proces}{{\text{s}}_{3\to 4}}\] are isochoric processes. \[{{W}_{12}}=0,\,{{W}_{34}}=0,\,{{W}_{23}}=nR({{T}_{3}}-{{T}_{2}})=3R(1600-400)\] \[=3600\,R\] \[{{W}_{41}}=nR({{T}_{1}}-{{T}_{4}})=3R(200-800)=-1800\,R\]You need to login to perform this action.
You will be redirected in
3 sec
