A) All I-Cl bonds are equivalent
B) Molecule is polar and non-planar
C) All adjacent bond angles are equal
D) All hybrid orbitals of central atom having equal s-character
Correct Answer: C
Solution :
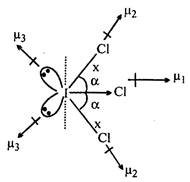 \[({{\mu }_{D}}\ne 0)\] \[x>y\] Molecule is polar and planar. Both \[\angle ClI\,Cl\] are equal Equatorial\[I-\,Cl\]bond has more s-character than axial \[I-\,Cl\]bond.
\[({{\mu }_{D}}\ne 0)\] \[x>y\] Molecule is polar and planar. Both \[\angle ClI\,Cl\] are equal Equatorial\[I-\,Cl\]bond has more s-character than axial \[I-\,Cl\]bond.
You need to login to perform this action.
You will be redirected in
3 sec
