A) 2 and 3
B) 3 and 3
C) 3 and 4
D) 4 and 3
Correct Answer: A
Solution :
[a] \[{{\mathbf{[Pt}\,{{\mathbf{(gly)}}_{\mathbf{2}}}\mathbf{]}}^{\mathbf{0}}}\mathbf{:}\] It is a square planar complex of \[[M{{(AB)}_{2}}]\] type in which \[gl{{y}^{\bigcirc -}}\] ion \[(N{{H}_{2}}.C{{H}_{2}}.CO{{O}^{\Theta }})\] is an unsymmetrical bidentate ligand. this complex has two geometrical isomers viz cis and trans.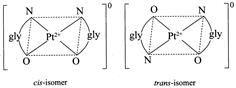 \[{{\mathbf{[Pt\{P(}{{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}}{{\mathbf{)}}_{\mathbf{3}}}\mathbf{\}C}{{\mathbf{l}}_{\mathbf{2}}}\mathbf{]}}_{\mathbf{2}}}\mathbf{:}\] It is a bridged binuclear planar complex and exists in three isomeric forms viz cis, trans and unsymmetrical.
\[{{\mathbf{[Pt\{P(}{{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}}{{\mathbf{)}}_{\mathbf{3}}}\mathbf{\}C}{{\mathbf{l}}_{\mathbf{2}}}\mathbf{]}}_{\mathbf{2}}}\mathbf{:}\] It is a bridged binuclear planar complex and exists in three isomeric forms viz cis, trans and unsymmetrical. 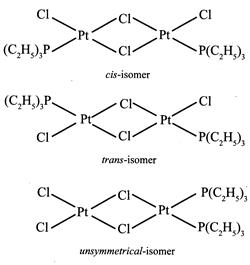
You need to login to perform this action.
You will be redirected in
3 sec
