A) \[0.6\overset{o}{\mathop{A}}\,\]
B) \[0.7\overset{o}{\mathop{A}}\,\]
C) \[0.8\overset{o}{\mathop{A}}\,\]
D) \[0.9\overset{o}{\mathop{A}}\,\]
Correct Answer: B
Solution :
[b]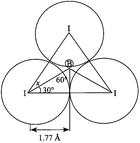 \[x={{d}_{IB}}=\frac{1.77}{\sin 60{}^\circ }=2.04\overset{o}{\mathop{A}}\,\] \[{{r}_{B}}=(2.04\overset{o}{\mathop{A}}\,)-(1.33\overset{o}{\mathop{A}}\,)=0.71\overset{o}{\mathop{A}}\,\]
\[x={{d}_{IB}}=\frac{1.77}{\sin 60{}^\circ }=2.04\overset{o}{\mathop{A}}\,\] \[{{r}_{B}}=(2.04\overset{o}{\mathop{A}}\,)-(1.33\overset{o}{\mathop{A}}\,)=0.71\overset{o}{\mathop{A}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
