 Select the INCORRECT statement.
Select the INCORRECT statement.
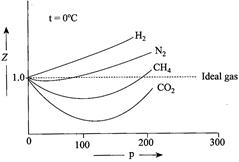
A) If \[b>a/RT,\]the initial slope is positive, and the size effect (i,e, 'b' factor) will dominate the behaviour of the gas.
B) If \[b<a/RT,\]the initial slope is negative, and the effect of the attractive forces (i,e, 'a' factor) will dominate the behavior of the gas.
C) At \[0{}^\circ C,\] the effect of attractive forces dominates the behaviour of \[C{{H}_{4}}\] and \[C{{O}_{2}}\] while the molecular size effect dominates the behaviour of \[{{H}_{2}}\]
D) If temperature is low enough, the term \[a/RT\]will be smaller than 'b" and so the initial slope of Z versus p will be positive. As the temperature rises,\[a/RT\].becomes larger. At a sufficiently high temperature it becomes more than 'b' and the initial slope of Z versus p becomes negative
Correct Answer: D
Solution :
[d] CORRECT statement: If the temperature is low enough the term a/RT will be larger than 'b' and so the initial slope of Z versus p will be negative. As the temperature rises, a/RT becomes smaller. At a sufficiently high temperature it becomes less than 'b' and the initial slope of Z versus p becomes positiveYou need to login to perform this action.
You will be redirected in
3 sec
