| The correct order of increasing basic nature of the following bases is |
| I. |
| II. |
| III. |
IV. 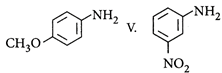 |
A) \[II<V<I<III<IV\]
B) \[V<II<I<III<IV\]
C) \[II<V<I<IV<III\]
D) \[V<II<I<IV<III\]
Correct Answer: A
Solution :
[a] : \[-OC{{H}_{3}}\]is strongest electron releasing group (+M effect) which opposes most the dispersion of lone pair of electrons of nitrogen into the ring. Thus, \[-OC{{H}_{3}}\]being at para position imparts highest basicity. \[-N{{O}_{2}}\]being at meta position stabilises the electron pair of nitrogen only by -I effect. While \[-N{{O}_{2}}\]being present at para position due to -M effect and -I effect stabilises the lone pair of electrons of nitrogen most and imparts least basicity.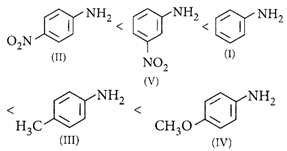 E.W.G. increases the acidity of benzoic acid, o-isomer will have higher acidity than corresponding m- and p-isomers due to ortho- effect. In p-nitro benzoic acid, both -R effect and -I effect of the nitro group increase the acidity while in m-nitro benzoic acid, only the weaker -I effect increases the acidity. Therefore the correct order of acidity is ii > iii > iv > i.
E.W.G. increases the acidity of benzoic acid, o-isomer will have higher acidity than corresponding m- and p-isomers due to ortho- effect. In p-nitro benzoic acid, both -R effect and -I effect of the nitro group increase the acidity while in m-nitro benzoic acid, only the weaker -I effect increases the acidity. Therefore the correct order of acidity is ii > iii > iv > i.
You need to login to perform this action.
You will be redirected in
3 sec
