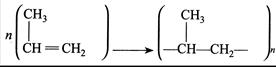
 Where n has large integral value, the average enthalpies of bond dissociation for \[(C=C)\] and \[(C-C)\] at 298 K are \[+590\] and\[+331\,kJmo{{l}^{-1}}\]. The enthalpy of polymerisation is\[-360\,kJ\,mo{{l}^{-1}}\]. Find the value of n.
Where n has large integral value, the average enthalpies of bond dissociation for \[(C=C)\] and \[(C-C)\] at 298 K are \[+590\] and\[+331\,kJmo{{l}^{-1}}\]. The enthalpy of polymerisation is\[-360\,kJ\,mo{{l}^{-1}}\]. Find the value of n.
A) 5
B) 10
C) 15
D) 20
Correct Answer: A
Solution :
[a] Energy released = Energy due to formation of two single bonds \[=2\times 331=661\,\,kJmo{{l}^{-1}}\]of propene \[\Delta H\]polymerisation / mol \[=590-662=-72\text{ }kJ\text{ }mo{{l}^{-1}}\] \[\Delta H\]polymerisation/mol \[=-72\times n=-360\] \[n=5\]You need to login to perform this action.
You will be redirected in
3 sec
