A) A - \[Mg\] B - \[M{{g}_{3}}{{N}_{2}}\] C - \[N{{H}_{3}}\]
B) A - \[Li\] B - \[L{{i}_{3}}N\] C - \[N{{H}_{3}}\]
C) A - \[Li\] B - \[L{{i}_{2}}O\] C - \[N{{O}_{2}}\]
D) A - \[Mg\] B - \[MgO\] C - \[N{{O}_{2}}\]
Correct Answer: B
Solution :
[b] In alkali metals only \[Li\]forms nitrides \[(L{{i}_{3}}N)\] with \[{{N}_{2}}\] (from air) whereas all alkaline earth metals forms nitrides (M N ). But \[Li\]gives crimson red flame colour whereas \[Be\]and \[Mg\]do not impart flame colour thus metal [a] is\[Li\].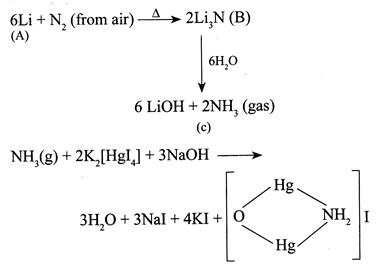 Iodide of millon's base (Brown ppt)
Iodide of millon's base (Brown ppt)
You need to login to perform this action.
You will be redirected in
3 sec
