A) \[-0.907V\]
B) \[0.907V\]
C) \[-0.313\]
D) \[0.313\]
Correct Answer: B
Solution :
[b]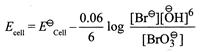 \[(pH=9.0,\,pOH=14-9=5,\,\overset{\bigcirc -}{\mathop{O}}\,H={{10}^{-5}}M\] Activity of \[{{H}_{2}}O=1\])
\[(pH=9.0,\,pOH=14-9=5,\,\overset{\bigcirc -}{\mathop{O}}\,H={{10}^{-5}}M\] Activity of \[{{H}_{2}}O=1\]) You need to login to perform this action.
You will be redirected in
3 sec
