A) A - \[CuS{{O}_{4}}.4{{H}_{2}}O\] B - \[CuS{{O}_{4}}\] C - \[CuO\] D - \[S{{O}_{3}}\]
B) A - \[CuS{{O}_{4}}.2{{H}_{2}}O\] B - \[CuS{{O}_{4}}.{{H}_{2}}O\] C - \[CuS{{O}_{4}}\] D - \[CuO\]
C) A - \[CuS{{O}_{4}}.3{{H}_{2}}O\] B - \[CuS{{O}_{4}}.\]\[2{{H}_{2}}O\] C - \[CuO\] D - \[S{{O}_{2}}\]
D) A - \[CuS{{O}_{4}}.{{H}_{2}}O\] B - \[CuO\] C - \[S{{O}_{3}}\] D - \[Cu{{O}_{2}}\]
Correct Answer: A
Solution :
[a]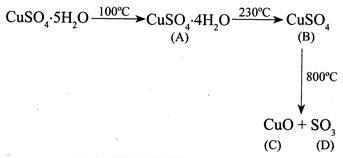
You need to login to perform this action.
You will be redirected in
3 sec
