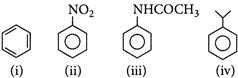
 The correct order towards electrophilic substitution reaction is
The correct order towards electrophilic substitution reaction is
A) (iv) > (iii) > (ii) > (i)
B) > (ii) > (iii) > (iv)
C) > (ii) > (i) > (iv)
D) > (iv) > (i) > (ii)
Correct Answer: D
Solution :
[d]: The more nucleophile compound among the given will undergo more rapid electrophilic substitution reaction. \[-NHCOC{{H}_{3}}\]group is more electron releasing group than \[-CH{{(C{{H}_{3}})}_{2}}\]group, exhibit more rapid electrophilic substitution reaction. Hence, the correct order is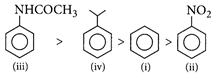
You need to login to perform this action.
You will be redirected in
3 sec
