| The P-V diagram of a system undergoint thermodynamic transformation is shown in figure. The work done on the system in going from \[A\to B\to C\] is \[50\text{ }J\]and 20 cat heat is given to the system. The change in internal energy between A and C is |
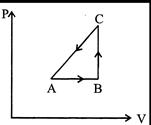 |
A) \[34\text{ }J\]
B) \[70\text{ }J\]
C) \[84\text{ }J\]
D) \[134J\]
Correct Answer: D
Solution :
Heat given \[\Delta Q=20\,cal=20\times 4.2=84J\] Work done \[\Delta W=-50\,J\][As process is anticlockwise] By first law of thermodynamics \[\Rightarrow \,\,\Delta U=\Delta Q-\Delta W=84-(-50)=134J\]You need to login to perform this action.
You will be redirected in
3 sec
