Answer:
(i)![]()
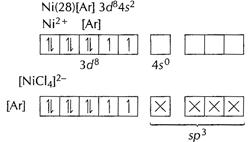
![]() is
a weak ligand, hence 3d' electrons remain unpaired. Geometry : tetrahedral.
Magnetic character paramagnetic due to unpaired
electrons
Magnetic moment
is
a weak ligand, hence 3d' electrons remain unpaired. Geometry : tetrahedral.
Magnetic character paramagnetic due to unpaired
electrons
Magnetic moment![]()
![]() [2]
(ii) Crystal field splitting in tetrahedral
[2]
(ii) Crystal field splitting in tetrahedral![]() (Crystal
field splitting in octahedral) Thus, magnitude of crystal field splitting in octahedral
crystal field is larger. [1]
(Crystal
field splitting in octahedral) Thus, magnitude of crystal field splitting in octahedral
crystal field is larger. [1]
You need to login to perform this action.
You will be redirected in
3 sec
