Answer:
(i) Phosphorous and (![]() ) is a
dibasic acid as shown in the following structure
) is a
dibasic acid as shown in the following structure
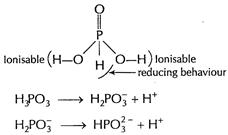 Basicity (= 2) is due to two ionisable
Basicity (= 2) is due to two ionisable ![]() attached
to O-atoms. H attached to P directly shows reducing behaviour.
[1]
(ii)
attached
to O-atoms. H attached to P directly shows reducing behaviour.
[1]
(ii)![]()
![]() fumes in
most air.
It is due to hydrolysis of
fumes in
most air.
It is due to hydrolysis of ![]() by the
moisture present in air. HCI is formed which fumes in air. [1]
(iii)
by the
moisture present in air. HCI is formed which fumes in air. [1]
(iii) ![]()
![]()
![]() At anode
At anode ![]() At cathode
At cathode ![]() Reaction between
Reaction between ![]() and
and ![]() is very
explosive of anodes and cathodes are not separated, violent reaction taken
place. [1]
is very
explosive of anodes and cathodes are not separated, violent reaction taken
place. [1]
You need to login to perform this action.
You will be redirected in
3 sec
